Unveiling the Heating and Cooling Curve Worksheet Answer Key, this guide embarks on a journey to illuminate the intricacies of this essential tool. Delve into the depths of heating and cooling curves, deciphering their significance in diverse scientific fields.
This comprehensive guide serves as a beacon of knowledge, empowering you to navigate the complexities of heating and cooling curve worksheets with ease. Prepare to unravel the mysteries of thermal transitions, gaining invaluable insights into the behavior of matter.
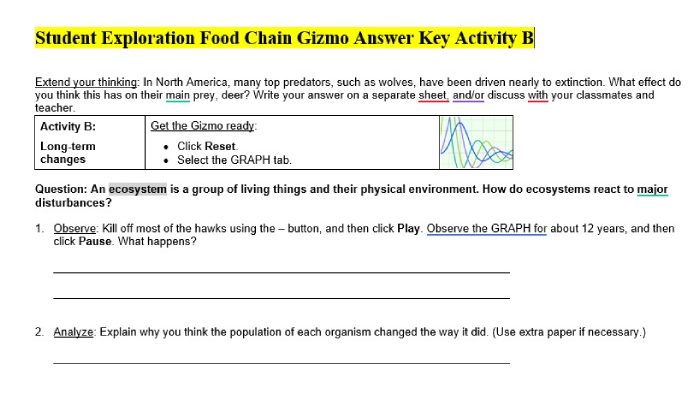
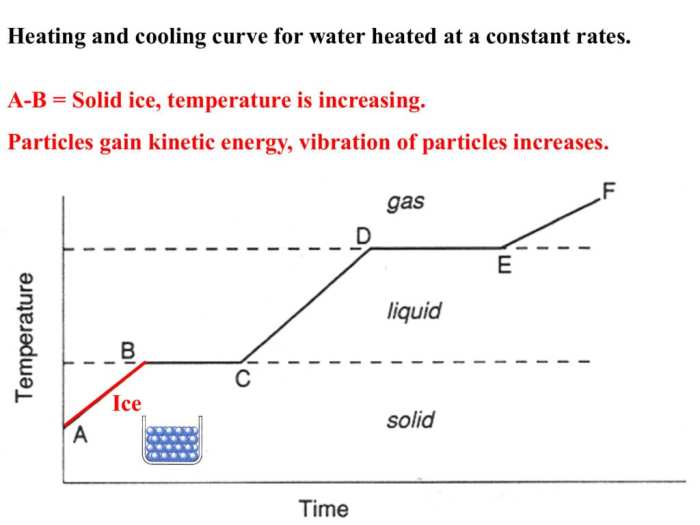
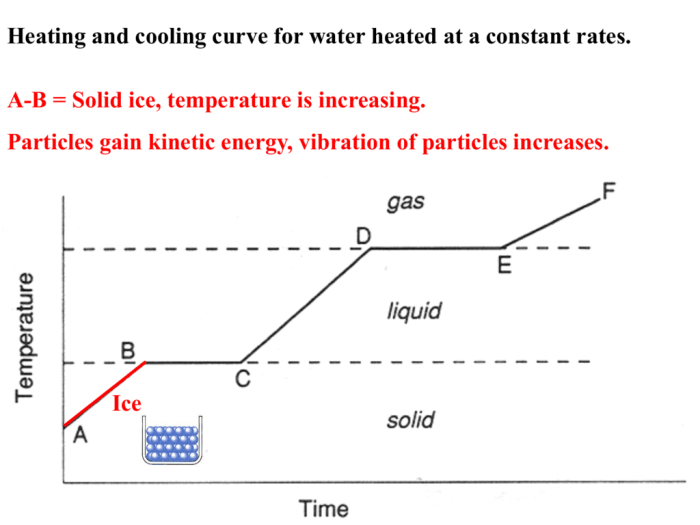
Heating and Cooling Curve
A heating and cooling curve is a graphical representation of the temperature of a substance as it undergoes heating or cooling. It provides valuable insights into the physical and chemical changes occurring within the substance.
The curve consists of distinct stages, each corresponding to a specific phase transition or change in the substance’s state. These stages include:
- Solid phase
- Melting
- Liquid phase
- Boiling
- Gas phase
The heating and cooling curve provides information about the substance’s:
- Melting point
- Boiling point
- Latent heat of fusion
- Latent heat of vaporization
- Specific heat capacity
Worksheet Answer Key: Heating And Cooling Curve Worksheet Answer Key
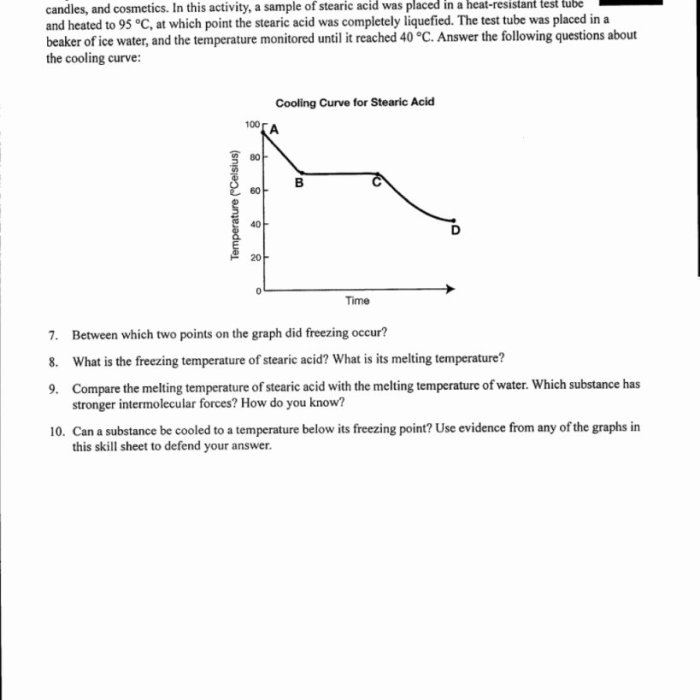
Heating and cooling curve worksheets typically include various types of questions. Here are some common questions and their explanations:
- Identify the stages of the heating and cooling curve.
- Determine the melting point and boiling point of the substance.
- Calculate the latent heat of fusion and vaporization.
- Explain the relationship between the slope of the curve and the specific heat capacity.
This question requires students to label the different sections of the curve, such as solid, liquid, and gas phases.
Students need to identify the temperatures at which the substance undergoes phase transitions.
These calculations involve determining the amount of energy required to change the substance’s phase.
The slope of the curve during the heating or cooling of a single phase represents the substance’s specific heat capacity.
Examples and Applications
Heating and cooling curves have numerous applications in various fields:
- Chemistry:Identifying unknown substances, determining purity, and studying phase transitions.
- Materials science:Investigating the thermal properties of materials, such as melting points and thermal conductivity.
- Engineering:Designing and optimizing heat transfer systems, such as boilers and condensers.
- Food science:Studying the effects of heating and cooling on food quality and safety.
- Medicine:Monitoring body temperature during surgery and diagnosing medical conditions related to temperature regulation.
Additional Resources
- Khan Academy: Heating and Cooling Curves
- Encyclopædia Britannica: Heating Curve
- PhET Interactive Simulation: States of Matter Basics
Popular Questions
What is the significance of heating and cooling curves?
Heating and cooling curves provide valuable insights into the thermal behavior of substances, revealing phase transitions, specific heat capacities, and enthalpy changes.
How can heating and cooling curves be used to solve problems?
By analyzing heating and cooling curves, scientists can determine unknown temperatures, identify substances, and calculate thermodynamic properties.
What are the different types of questions found on heating and cooling curve worksheets?
Worksheets may include questions on identifying phase transitions, calculating enthalpy changes, and interpreting temperature-time graphs.