Chemical and physical change webquest – Embark on a captivating expedition into the realm of chemical and physical change with this comprehensive webquest. Immerse yourself in a world of transformations, where substances undergo alterations in their composition and properties. Delve into the intricacies of chemical reactions and witness the marvels of physical changes, gaining a profound understanding of the fundamental processes that shape our world.
From the subtle shifts in physical states to the profound rearrangements of chemical bonds, this webquest unveils the secrets behind the myriad changes that occur in our surroundings. Prepare to unravel the mysteries of matter and uncover the fascinating interplay between its chemical and physical properties.
Chemical and Physical Change Definitions

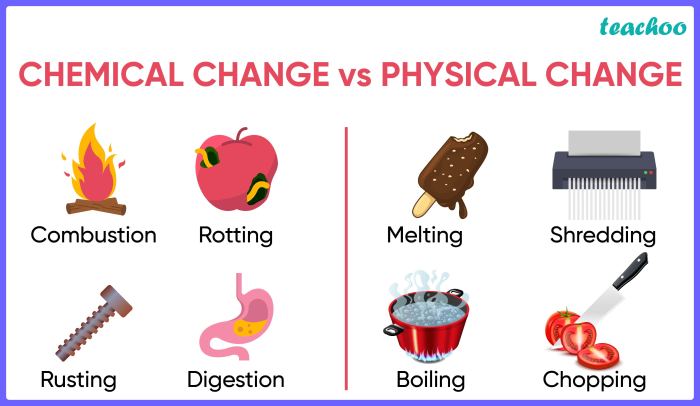
Chemical and physical changes are two distinct types of changes that can occur to matter. Chemical changes involve the rearrangement of atoms to form new substances, while physical changes involve changes in the physical properties of a substance without changing its chemical composition.
Chemical Change
A chemical change is a change that results in the formation of a new substance. This can occur when atoms are rearranged to form new molecules, or when electrons are transferred from one atom to another. Chemical changes are often accompanied by changes in energy, such as the release of heat or light.
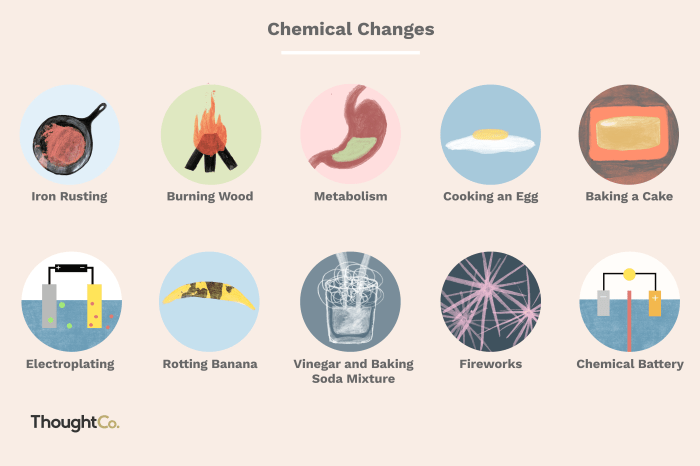
Examples of chemical changes include:
- Burning of wood
- Rusting of iron
- Cooking of food
- Photosynthesis
Physical Change
A physical change is a change that does not result in the formation of a new substance. This can occur when a substance changes its physical properties, such as its shape, size, or state of matter. Physical changes are not accompanied by changes in energy.
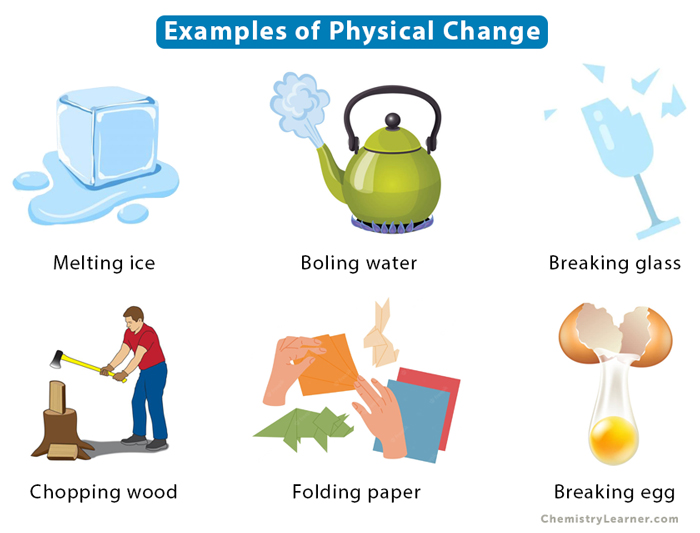
Examples of physical changes include:
- Melting of ice
- Boiling of water
- Condensation of steam
- Dissolving of sugar in water
Types of Chemical Changes

Chemical changes involve the rearrangement of atoms to form new substances with different properties. These changes can be classified into several types based on the nature of the reaction.
Combination Reactions
Combination reactions occur when two or more simple substances combine to form a more complex compound. For example, hydrogen and oxygen combine to form water:
2H2+ O 2→ 2H 2O
Decomposition Reactions
Decomposition reactions are the opposite of combination reactions. They involve the breakdown of a compound into simpler substances. For example, water can be decomposed into hydrogen and oxygen:
2H2O → 2H 2+ O 2
Single-Replacement Reactions
Single-replacement reactions involve the replacement of one element in a compound by another element. For example, iron can replace copper in copper sulfate to form iron sulfate and copper:
Fe + CuSO4→ FeSO 4+ Cu
Double-Replacement Reactions
Double-replacement reactions involve the exchange of ions between two compounds. For example, sodium chloride and silver nitrate can react to form sodium nitrate and silver chloride:
NaCl + AgNO3→ NaNO 3+ AgCl
Types of Physical Changes
Physical changes involve changes in the form or appearance of a substance without altering its chemical composition. These changes are typically reversible, meaning that the substance can be returned to its original form.
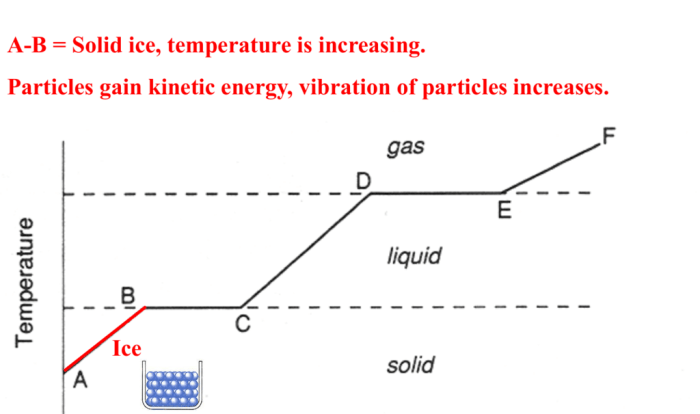
Melting
Melting is the physical change of a solid substance turning into a liquid when heated. This occurs when the temperature of the solid increases, causing the particles to gain energy and overcome the attractive forces holding them in a fixed position.
As a result, the particles become more mobile and can flow past each other, resulting in the formation of a liquid.Examples:
- Ice melting into water
- Butter melting into a liquid
- Chocolate melting when heated
Freezing
Freezing is the opposite of melting and involves the transformation of a liquid into a solid when cooled. As the temperature of the liquid decreases, the particles lose energy and slow down. This leads to a decrease in the kinetic energy of the particles, causing them to lose their ability to move freely.
As a result, the particles become more closely packed and form a rigid structure, resulting in the formation of a solid.Examples:
- Water freezing into ice
- Molten lava cooling and solidifying into rock
- Liquid nitrogen freezing into a solid
Sublimation
Sublimation is a physical change where a solid substance directly transforms into a gas without passing through the liquid phase. This occurs when the temperature and pressure conditions are such that the solid particles gain enough energy to overcome the attractive forces holding them together and directly enter the gaseous state.Examples:
- Dry ice (solid carbon dioxide) subliming into carbon dioxide gas
- Mothballs (solid naphthalene) subliming into naphthalene gas
- Iodine crystals subliming into iodine vapor
Condensation, Chemical and physical change webquest
Condensation is the opposite of sublimation and involves the transformation of a gas into a liquid. This occurs when the temperature of the gas decreases, causing the particles to lose energy and slow down. As a result, the kinetic energy of the particles decreases, leading to a decrease in their ability to move freely.
This allows the particles to come closer together and form intermolecular bonds, resulting in the formation of a liquid.Examples:
- Water vapor condensing into liquid water
- Steam condensing on a cold surface
- Formation of dew on grass in the morning
Vaporization
Vaporization is a general term used to describe the physical change where a liquid or solid substance transforms into a gas. This can occur through either evaporation or boiling. Evaporation is the process where a liquid transforms into a gas at temperatures below its boiling point, while boiling is the process where a liquid transforms into a gas at its boiling point.Examples:
- Water evaporating from a puddle
- Alcohol evaporating from a solution
- Liquid nitrogen boiling into nitrogen gas
Factors Affecting Chemical and Physical Changes
Chemical and physical changes are influenced by various factors that can alter their rate and extent. Understanding these factors is crucial for controlling and predicting the outcome of chemical and physical processes.
Temperature
Temperature plays a significant role in both chemical and physical changes. Increased temperature generally increases the rate of chemical reactions, as higher temperatures provide more energy for reactant molecules to overcome the activation energy barrier. In physical changes, temperature can cause substances to melt, vaporize, or expand.
For example, ice melts into water when heated, and a gas expands when its temperature rises.
Pressure
Pressure can influence chemical and physical changes in gases and liquids. Increased pressure can favor reactions that produce fewer gas molecules, while decreased pressure can favor reactions that produce more gas molecules. In physical changes, pressure can affect the boiling point of liquids and the melting point of solids.
For instance, water boils at a lower temperature at higher altitudes due to decreased atmospheric pressure.
Concentration
Concentration, specifically the concentration of reactants, affects the rate of chemical reactions. Higher concentrations of reactants lead to a higher probability of collisions between reactant molecules, resulting in a faster reaction rate. In physical changes, concentration can influence the rate of diffusion and the solubility of substances.
For example, a sugar cube dissolves faster in hot water than in cold water due to the higher concentration of sugar molecules in the hot water.
Surface Area
Surface area is a crucial factor in both chemical and physical changes involving solids. Increased surface area provides more contact points for reactions to occur. For example, a powdered metal reacts faster than a solid chunk of the same metal because the powder has a larger surface area.
In physical changes, increased surface area can enhance the rate of evaporation or sublimation. For instance, a wet cloth dries faster when spread out than when bunched up.
Applications of Chemical and Physical Changes
Chemical and physical changes are pervasive in our daily lives, with applications spanning various fields and industries. These changes underlie numerous processes and phenomena, enabling us to harness the properties of matter for practical purposes.
Applications of Chemical Changes
Chemical changes involve the transformation of one set of substances into a new set of substances with distinct chemical compositions and properties. These changes play a crucial role in:
- Energy Production:Combustion reactions, such as burning fossil fuels, release energy that powers engines and generates electricity.
- Food Processing:Cooking, baking, and fermentation are chemical processes that transform raw ingredients into palatable and nutritious foods.
- Pharmaceuticals:Chemical synthesis is essential for producing medicines and vaccines to treat diseases.
- Materials Science:Chemical changes enable the creation of new materials with enhanced properties, such as strength, durability, and conductivity.
Applications of Physical Changes
Physical changes involve changes in the physical properties of matter, such as shape, volume, and density, without altering its chemical composition. These changes find applications in:
- Phase Transitions:Melting, freezing, boiling, and sublimation are physical changes used in processes such as refrigeration, air conditioning, and water purification.
- Separation Techniques:Filtration, distillation, and chromatography are physical methods used to separate mixtures based on their physical properties.
- Materials Processing:Rolling, forging, and extrusion are physical changes that shape and form metals and other materials.
- Energy Storage:Physical changes, such as phase transitions and adsorption, are utilized in energy storage systems, such as batteries and fuel cells.
Lab Activities for Chemical and Physical Changes
Lab activities provide hands-on experiences that help students understand the concepts of chemical and physical changes. These activities allow students to observe and analyze the changes that occur in substances and to identify the factors that influence these changes.
Chemical Change Lab Activity
Objective:To demonstrate a chemical change by observing the reaction between baking soda and vinegar.
Materials:* Baking soda
- Vinegar
- Clear glass or plastic container
- Measuring cups and spoons
Procedure:
- Measure 1/4 cup of baking soda into the container.
- Measure 1/2 cup of vinegar into a separate container.
- Slowly add the vinegar to the baking soda.
- Observe the reaction that occurs.
Expected Observations:* Formation of bubbles
- Release of carbon dioxide gas
- Change in temperature
- Change in color
Physical Change Lab Activity
Objective:To demonstrate a physical change by observing the melting of ice.
Materials:* Ice cubes
- Glass or plastic container
- Heat source (e.g., hot plate, microwave)
Procedure:
- Place several ice cubes in the container.
- Heat the container until the ice cubes melt.
- Observe the changes that occur.
Expected Observations:* Change in state from solid to liquid
- No change in chemical composition
- Reversible change
Popular Questions: Chemical And Physical Change Webquest
What is the difference between chemical and physical change?
Chemical change involves a rearrangement of atoms to form new substances, while physical change alters the form or appearance of a substance without changing its chemical composition.
Can physical changes be reversed?
Yes, most physical changes are reversible, such as melting and freezing. However, some physical changes, like sublimation, may not be easily reversed.
What are some examples of chemical changes in everyday life?
Cooking, burning, and rusting are common examples of chemical changes that occur in our daily lives.